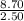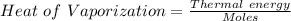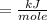Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, PSBSolarYT
For which one of the following reactions is the value of δh° rxn equal to δh° f for the product? a. 2 h2 (g) + o2 (g) → 2 h2o (l) b. n2 (g) + o2 (g) → 2 no (g) c. 2 h2 (g) + o2 (g) → 2 h2o (g) d. h2o (l) + 1/2 o2 (g) → h2o2 (l) e. none of the above
Answers: 1

Chemistry, 22.06.2019 01:30, elizediax8683
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 04:30, aleilyg2005
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
When 8.70 kj of thermal energy is added to 2.50 mol of liquid methanol, it vaporizes. determine the...
Questions in other subjects:



History, 12.10.2020 07:01



Mathematics, 12.10.2020 07:01



Health, 12.10.2020 07:01