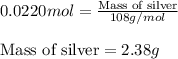Zinc metal reacts with silver nitrate according to the reaction:
zn(s) + 2agno3(aq)zn(n...

Chemistry, 24.01.2020 05:31 scarlettp13
Zinc metal reacts with silver nitrate according to the reaction:
zn(s) + 2agno3(aq)zn(no3 )2 (aq) + 2ag(s)
calculate the mass of ag that forms when 3.00g of zinc metal is placed in an aqueous solution containing 3.75g of silver nitrate?

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:30, pup88
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 15:30, ricardotavarez6
How does a large body of water, such as the ocean, influence climate?
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 25.09.2021 01:00

Spanish, 25.09.2021 01:00


Mathematics, 25.09.2021 01:00


Computers and Technology, 25.09.2021 01:00


English, 25.09.2021 01:00

Mathematics, 25.09.2021 01:00

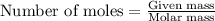 ....(1)
....(1)
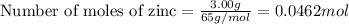
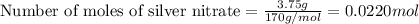
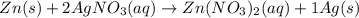
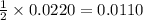 moles of zinc
moles of zinc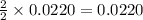 moles of silver
moles of silver