
Chemistry, 24.01.2020 04:31 bbbbriasutton
Which solution is more concentrated?
a. 2.00 moles of naoh dissolved in 1.00 liter of solution
b. 2.00 grams of naoh dissolved in 1.00 liter of solution

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 23.06.2019 09:00, student0724
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2


You know the right answer?
Which solution is more concentrated?
a. 2.00 moles of naoh dissolved in 1.00 liter of s...
a. 2.00 moles of naoh dissolved in 1.00 liter of s...
Questions in other subjects:



History, 03.02.2021 20:30

Chemistry, 03.02.2021 20:30

English, 03.02.2021 20:30





English, 03.02.2021 20:30

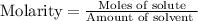
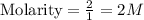
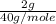 which is equal to 0.05 moles of NaOH.
which is equal to 0.05 moles of NaOH.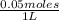 =0.05 M
=0.05 M

