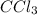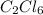Chemistry, 24.01.2020 02:31 hqlego6882
Acompound contains 10.13% c and 89.87% cl (by mass). determine both the empirical formula and the molecular formula of the compound given that the molar mass is 237 g/mol.
ccl3
c2cl
ccl

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, yasiroarafat12
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 16:00, matt16913
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 22:30, creepycrepes
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1
You know the right answer?
Acompound contains 10.13% c and 89.87% cl (by mass). determine both the empirical formula and the mo...
Questions in other subjects:

History, 16.07.2019 13:00

Mathematics, 16.07.2019 13:00


Mathematics, 16.07.2019 13:00


Biology, 16.07.2019 13:00

Mathematics, 16.07.2019 13:00