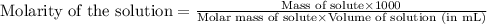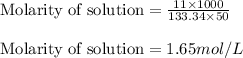Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 03:30, alecnewman2002
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1
You know the right answer?
Achemist prepares a solution of aluminum chloride by measuring out of aluminum chloride into a volum...
Questions in other subjects:


Social Studies, 18.08.2019 09:00


Mathematics, 18.08.2019 09:00


Mathematics, 18.08.2019 09:00

World Languages, 18.08.2019 09:00



History, 18.08.2019 09:00