
Chemistry, 23.01.2020 22:31 ashtonlauber95
Suppose 0.10 mol of cu(no_3)_2 and 1.50 mol of nh_3 are dissolved in water and diluted to a total volume of 1.00 l. calculate the concentrations of cu(nh_3)_4^2+ and of cu^2+ at equilibrium.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:30, DragonLovely
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 02:00, jacckiie5176
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 05:40, girlchamp654
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2
You know the right answer?
Suppose 0.10 mol of cu(no_3)_2 and 1.50 mol of nh_3 are dissolved in water and diluted to a total vo...
Questions in other subjects:

Chemistry, 16.12.2019 07:31


Mathematics, 16.12.2019 07:31

Spanish, 16.12.2019 07:31



History, 16.12.2019 07:31

History, 16.12.2019 07:31

Mathematics, 16.12.2019 07:31

History, 16.12.2019 07:31

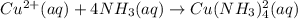
![K_{f} = \frac{[Cu(NH3)^{2+}_{4}]}{[Cu^{2+}][NH_{3}]_{4}}](/tpl/images/0467/9077/53418.png)
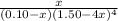
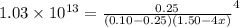
![[NH_{3}] = 1.50 - 4x = (\frac{2.33}{1.03 \times 10^{13}})^{\frac{1}{4}](/tpl/images/0467/9077/925c0.png)
![[Cu(NH_{3})^{2+}_{4}]](/tpl/images/0467/9077/dbe3c.png)
![\frac{1.50 - 2.31284 \times 10{-4}}{4}]](/tpl/images/0467/9077/19dce.png)
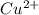 ) is 0.37491425 M.
) is 0.37491425 M.

