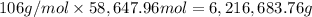Atanker truck carrying 6.05×103 kg of concentrated sulfuric acid solution tips over and spills its load. the sulfuric acid solution is 95.0%h2so4 by mass and has a density of 1.84 g/ml.
part a
sodium carbonate (na2co3) is used to neutralize the sulfuric acid spill. how many kilograms of sodium carbonate must be added to neutralize 6.05×103 kg of sulfuric acid solution?
express your answer with the appropriate units

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, sheazy3709
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2


Chemistry, 23.06.2019 05:00, neidaq12345
110 g of water (specific heat = 4.184 j/g c) and 100 g of a metal sample (specific heat = 0.397 j/g c) are heated from 25 degrees c to 75 degrees c. which substance required more thermal energy?
Answers: 1
You know the right answer?
Atanker truck carrying 6.05×103 kg of concentrated sulfuric acid solution tips over and spills its l...
Questions in other subjects:


Computers and Technology, 07.05.2020 04:08




Advanced Placement (AP), 07.05.2020 04:08




Mathematics, 07.05.2020 04:08

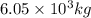 of sulfuric acid solution.
of sulfuric acid solution.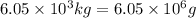
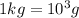
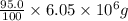
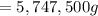
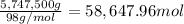
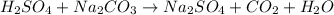
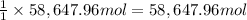 of sodium carbonate
of sodium carbonate