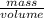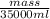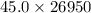Agram of gasoline produces 45.0 kj of energy when burned. gasoline has a density of 0.77 g/ml. how would you ca the amount of energy produced by burning 35. l of gasoline? set the math up. but don't do any of it. just leave your answer as a math expression. also, be sure your answer includes all the correct unit symbols.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1


Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2
You know the right answer?
Agram of gasoline produces 45.0 kj of energy when burned. gasoline has a density of 0.77 g/ml. how w...
Questions in other subjects:

Chemistry, 28.08.2019 15:30


Mathematics, 28.08.2019 15:30

World Languages, 28.08.2019 15:30



Mathematics, 28.08.2019 15:30


Social Studies, 28.08.2019 15:30

Mathematics, 28.08.2019 15:30