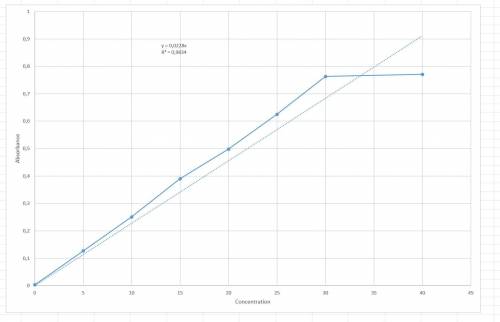
Chemistry, 23.01.2020 05:31 lraesingleton
The following results were obtained when each of a series of standard silver solutions was analyzed by flame-atomic absorption spectrometry: concentration on 0 5 10 15 20 25 30 40 (ng. ml1) absorbance(r. u.) 0.003 0.127 0.251 0.390 0.498 0.625 0.763 0.771 determine the slope and intercept of the calibration plot, along with their confidence limits (95%). using the data from exercise 1 above, estimate the confidence limits for the silver concentration in: a) a sample giving an absorbance of 0.456 in a single determination. b) a sample giving absorbance values of 0.308, 0.317, 0.347, and 0.412 in four separate determinations. graph: a straight line excel plot is shown in the question

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:50, khorasanpublic
The number at the end of an isotope’s name is the number.
Answers: 1


Chemistry, 22.06.2019 20:40, ohgeezy
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1
You know the right answer?
The following results were obtained when each of a series of standard silver solutions was analyzed...
Questions in other subjects:

English, 30.11.2021 20:50




Mathematics, 30.11.2021 20:50

Mathematics, 30.11.2021 20:50