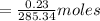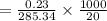Chemistry, 23.01.2020 05:31 lovexoxdivap0ifhi
Apure organic amide product (2.85 g) which has a molecular weight of 285.34 g/mol was fully dissolved in 20ml of the boiling solvent, hexanes. this organic amide product then underwent a recrystallization when the solvent was cooled to 0° c. the recrystallized organic product (2.62 grams) was obtained after vacuum filtration. what is the solubility of the organic amide product in the solvent, hexanes, at 0° c?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 15:40, alleshia2007
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 18:00, jeepjose58
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

You know the right answer?
Apure organic amide product (2.85 g) which has a molecular weight of 285.34 g/mol was fully dissolve...
Questions in other subjects:




History, 20.10.2020 02:01





Business, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01