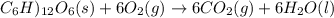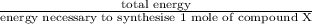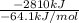Chemistry, 23.01.2020 03:31 coleman310
Assume that the complete combustion of one mole of fructose, a monosaccharide, to carbon dioxide and water liberates 2810 kj (δg°\' = –2810 kj/mol). if the energy generated by the combustion of fructose is entirely converted to the synthesis of a hypothetical compound x, calculate the number of moles of the compound that could theoretically be generated. use the value δg°\'compound x = − 64.1 kj/mol kj/mol. round your answer to two significant figures.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, shaylawaldo11
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1


Chemistry, 23.06.2019 04:20, vliu732
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
Assume that the complete combustion of one mole of fructose, a monosaccharide, to carbon dioxide and...
Questions in other subjects:




Biology, 15.07.2019 07:00

Mathematics, 15.07.2019 07:00

Computers and Technology, 15.07.2019 07:00