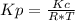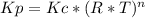Chemistry, 23.01.2020 03:31 bakaoffire
Consider the following chemical equilibrium: c(s) +2h2 (g) ch4 (g) now write an equation below that shows how to calculate from for this reaction at an absolute temperature . you can assume is comfortably above room temperature. if you include any common physical constants in your equation be sure you use their standard symbols, found in the aleks calculator.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:10, babyphoraaaaa
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate, m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

You know the right answer?
Consider the following chemical equilibrium: c(s) +2h2 (g) ch4 (g) now write an equation below that...
Questions in other subjects:





Health, 18.10.2020 15:01




Mathematics, 18.10.2020 15:01