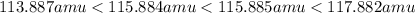The two naturally occurring isotopes of bromine are
81br (80.916 amu, 49.31%) and
79br (78.918 amu, 50.69%).
the two naturally occurring isotopes of chlorine are
37cl (36.966 amu, 24.23%) and
35cl (34.969 amu, 75.77%).
bromine and chlorine combine to form bromine monochloride, brcl.
what are the masses of the four different brcl molecules? express the masses in atomic mass units using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:00, sophiapknight
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 21:30, kawaiiblurainbow
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
The two naturally occurring isotopes of bromine are
81br (80.916 amu, 49.31%) and
<...
81br (80.916 amu, 49.31%) and
<...
Questions in other subjects:

Mathematics, 09.07.2021 18:50









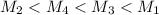

 = 80.916 amu + 36.966 amu
= 80.916 amu + 36.966 amu  = 78.918 amu + 34.969 amu
= 78.918 amu + 34.969 amu 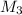 = 80.916 amu + 34.969 amu
= 80.916 amu + 34.969 amu  = 78.918 amu + 36.966 amu
= 78.918 amu + 36.966 amu