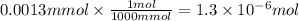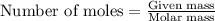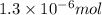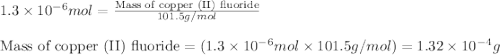Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, rosieposie27
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1


Chemistry, 23.06.2019 03:00, duplessistoccara
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2
You know the right answer?
Achemist adds of a m copper(ii) fluoride solution to a reaction flask. calculate the mass in microgr...
Questions in other subjects:

Geography, 23.02.2021 16:50

Mathematics, 23.02.2021 16:50

Business, 23.02.2021 16:50






Mathematics, 23.02.2021 16:50

English, 23.02.2021 16:50

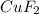 solution to a reaction flask.
solution to a reaction flask.