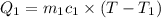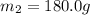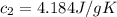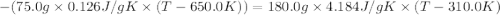Chemistry, 22.01.2020 20:31 justin5163
A75.0 g piece of gold at 650. k is dropped into 180. g of h2o(l) at 310. k in an insulated container at 1 bar pres- sure. calculate the temperature of the system once equilib- rium has been reached. assume that cp, m for au and h2o is constant at their values for 298 k throughout the temperature range of interest.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 11:10, hannah2757
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 14:30, amylumey2005
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
A75.0 g piece of gold at 650. k is dropped into 180. g of h2o(l) at 310. k in an insulated container...
Questions in other subjects:







Mathematics, 07.04.2021 16:50

Spanish, 07.04.2021 16:50


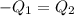
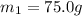
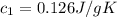
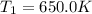
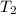 =T
=T