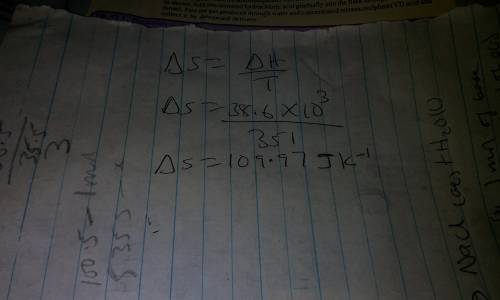Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:20, blondielocks2002
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 22:00, huddyxo
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 22.06.2019 23:00, DESI111609
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 23.06.2019 08:40, mathisaqeosmw
Calculate the number of grams of sodium in 3.00 g of each sodium-containing food additive.
Answers: 3
You know the right answer?
The boiling point of ethanol is 78 °c. the molar heat(i. e., enthalpy)of vaporization of ethanol is...
Questions in other subjects: