
Chemistry, 22.01.2020 04:31 carolinerosewillis
Asolution is prepared by dissolving 38.6 g sucrose (c12h22o11) in 495 g of water. determine the mole fraction of sucrose if the final volume of the solution is 508 ml.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, ayoismeisalex
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2


Chemistry, 22.06.2019 10:00, micahwilkerson9495
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2
You know the right answer?
Asolution is prepared by dissolving 38.6 g sucrose (c12h22o11) in 495 g of water. determine the mole...
Questions in other subjects:






Mathematics, 14.01.2020 20:31



Mathematics, 14.01.2020 20:31

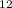 H
H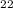 O
O = 144 + 22 + 176 = 342 g/molGiven Mass of C
= 144 + 22 + 176 = 342 g/molGiven Mass of C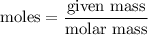
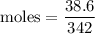
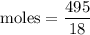
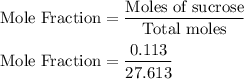 Mole fraction of sucrose = 0.004
Mole fraction of sucrose = 0.004


