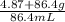Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3


Chemistry, 22.06.2019 22:30, jaylenmiller437
The diagram shows the relationship between scientific disciplines. the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a. physics b. biology c. chemistry d. metallurgy
Answers: 2
You know the right answer?
Asolution is made by dissolving 4.87 g of potassium nitrate in water to a final volume of 86.4 ml so...
Questions in other subjects:


English, 07.10.2020 05:01




Mathematics, 07.10.2020 05:01