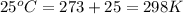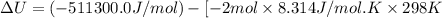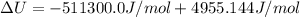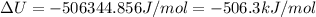Chemistry, 22.01.2020 04:31 george6871
The standard internal energy change for a reaction can be symbolized as δ u ∘ rxn or δ e ∘ rxn . for each reaction equation, calculate the energy change of the reaction at 25 ∘ c and 1.00 bar . sn ( s ) + 2 cl 2 ( g ) ⟶ sncl 4 ( l ) δ h ∘ rxn = − 511.3 kj/mol

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:10, PineaPPle663
Nuclear fusion is the source of energy for stars. besides hydrogen, which other element is most likely also common in stars?
Answers: 1

Chemistry, 21.06.2019 20:40, ellemarshall13
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 07:50, carlosiscr7
Many reactions take place in aqueous solution. when potential reactants are mixed, a reaction will occur if there is some driving force that favors the formation of products. it is often convenient to categorize reactions in terms of these driving forces: precipitate formation, in which an insoluble solid is formed, weak electrolyte formation, as in a neutralization reaction involving water, or transfer of electrons, as in a redox reaction. these reactions can be represented by full molecular equations, which contain all species in the reaction mixture, or by net ionic equations, which show only the species that actually undergo a change. the latter does not contain the spectator ions, which do not undergo a net change or do not take part in the reaction. part a when the following two solutions are mixed: k2co3(aq)+fe(no3)3(aq) the mixture contains the ions listed below. sort these species into spectator ions and ions that react. drag the appropriate items to their respective bins. view available hint(s) spectator ions ions that react part b what is the correct net ionic equation, including all coefficients, charges, and phases, for the following set of reactants? assume that the contribution of protons from h2so4 is near 100 %.ba(oh)2(aq)+h2so4(aq)→ express your answer as a chemical equation. view available hint(s) nothing provide feedback
Answers: 3
You know the right answer?
The standard internal energy change for a reaction can be symbolized as δ u ∘ rxn or δ e ∘ rxn . for...
Questions in other subjects:

Spanish, 09.02.2021 19:10

English, 09.02.2021 19:10


Mathematics, 09.02.2021 19:10

Chemistry, 09.02.2021 19:10

Chemistry, 09.02.2021 19:10

Mathematics, 09.02.2021 19:10

History, 09.02.2021 19:10

Mathematics, 09.02.2021 19:10

History, 09.02.2021 19:10

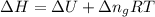
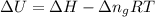
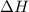 = change in enthalpy =
= change in enthalpy = 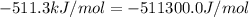
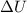 = change in internal energy = ?
= change in internal energy = ?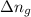 = change in moles
= change in moles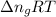 = 0
= 0