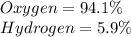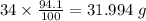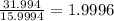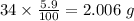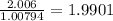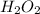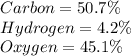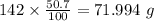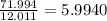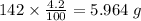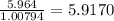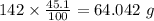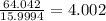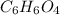Determine the molecular formula for each compound.
a) 94.1% oxygen and 5.9% hydrogen; molar mass = 34g
b) 50.7% carbon, 4.2% hydrogen, and 45.1% oxygen; molar mass = 142g
(would greatly appreciated if someone could explain the process, also use the correct amount of significant digits)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, BLASIANNkidd
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1

Chemistry, 22.06.2019 02:50, jordan5778
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1
You know the right answer?
Determine the molecular formula for each compound.
a) 94.1% oxygen and 5.9% hydrogen; molar...
a) 94.1% oxygen and 5.9% hydrogen; molar...
Questions in other subjects:

Mathematics, 02.10.2020 20:01

Biology, 02.10.2020 20:01


Chemistry, 02.10.2020 20:01


Mathematics, 02.10.2020 20:01


Medicine, 02.10.2020 20:01


Mathematics, 02.10.2020 20:01

 molecules.
molecules.