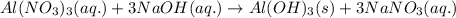Chemistry, 21.01.2020 22:31 quanyacoles124
Write a balanced equation for each of the following reactions include the state of each reactant and product. then identify the reaction type for each. are mixed, solid aluminum when aluminum nitrate and hydroxide forms. the other product is sodium nitrate.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, vivianfling
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1


Chemistry, 22.06.2019 20:30, ShahinF7536
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
Write a balanced equation for each of the following reactions include the state of each reactant and...
Questions in other subjects:

Mathematics, 17.06.2020 08:57





Mathematics, 17.06.2020 08:57

Chemistry, 17.06.2020 08:57


Biology, 17.06.2020 08:57