
Chemistry, 21.01.2020 22:31 edgartorres5123
According to a label on a bottle of concentrated hydrochloric acid, the contents are 36.0% hcl by mass and have a density of 1.18 g/ml.
1. what is the molarity of concentrated hcl.
2. what volume of it would you need to prepare 985 ml of 1.6 m hcl?
3. what mass of sodium bicarbonate would be needed to neutralize the spill if a bottle containing 1.75 l of concentrated hcl dropped on a lab floor and broke open?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

You know the right answer?
According to a label on a bottle of concentrated hydrochloric acid, the contents are 36.0% hcl by ma...
Questions in other subjects:

Biology, 10.07.2019 20:30

Mathematics, 10.07.2019 20:30

Biology, 10.07.2019 20:30







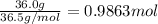
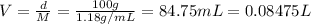
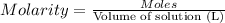
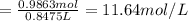
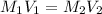 ( Dilution equation)
( Dilution equation)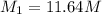
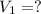
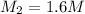
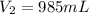
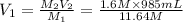
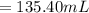
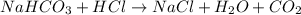
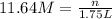
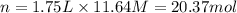
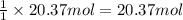 of sodium carbonate
of sodium carbonate 

