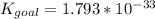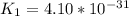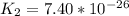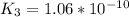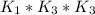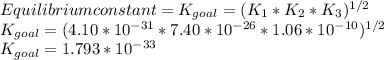Chemistry, 21.01.2020 06:31 joelpimentel
Determine the value of the equilibrium constant, kgoal, for the reactionn2(g)+h2o(g)⇌no(g)+12n2h4(g ), kgoal=? by making use of the following information: 1. n2(g)+o2(g)⇌2no(g), k1 = 4.10×10−312. n2(g)+2h2(g)⇌n2h4(g), k2 = 7.40×10−263. 2h2o(g)⇌2h2(g)+o2(g), k3 = 1.06×10−10express your answer numerically.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, cbelew0001ouje4i
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1


Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
Determine the value of the equilibrium constant, kgoal, for the reactionn2(g)+h2o(g)⇌no(g)+12n2h4(g...
Questions in other subjects:


Mathematics, 26.04.2021 22:00



Physics, 26.04.2021 22:00


Mathematics, 26.04.2021 22:00

Computers and Technology, 26.04.2021 22:00


History, 26.04.2021 22:00