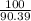Chemistry, 21.01.2020 05:31 fangkronos
Suppose you have a 100-gram sample of each of the following compounds. which sample contains the largest number of moles of compound? chromium(iii) chlorideammoniamagnesium chloridephosphoric acidpotassium chloridenitrogen chlorate

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, tamiaollie
1. baking powder is a 1: 1 molar mixture of cream of tartar (khc4h4o6) and baking soda (nahco3). a recipe calls for two teaspoons (a total of 8.0 grams) of cream of tartar. how much baking soda must be added for both materials to react completely?
Answers: 2

Chemistry, 22.06.2019 06:30, reecedstceklein
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 12:30, pup88
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
Suppose you have a 100-gram sample of each of the following compounds. which sample contains the lar...
Questions in other subjects:


Mathematics, 03.07.2019 04:30

Mathematics, 03.07.2019 04:30

English, 03.07.2019 04:30


English, 03.07.2019 04:30


Biology, 03.07.2019 04:30


Mathematics, 03.07.2019 04:30

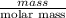
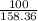
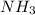 =
= 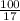
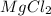 =
= 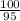
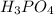 =
= 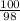
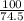
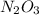 =
= 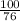
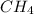 =
= 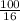
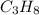 =
= 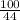
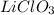 =
=