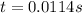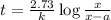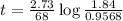At 1000°c, cyclobutane (c4h8) decomposes in a first-order reaction, with the very high rate constant of 68 1/s, to two molecules of ethylene (c2h4). the initial cyclobutane concentration is 1.84. how long will it take for 52% of the cyclobutane to decompose? enter to 4 decimal places.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2

Chemistry, 23.06.2019 04:20, milkshakegrande101
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1

Chemistry, 23.06.2019 08:00, mantha0402
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 1
You know the right answer?
At 1000°c, cyclobutane (c4h8) decomposes in a first-order reaction, with the very high rate constant...
Questions in other subjects:



Computers and Technology, 20.01.2020 00:31

History, 20.01.2020 00:31


Mathematics, 20.01.2020 00:31

Physics, 20.01.2020 00:31


Social Studies, 20.01.2020 00:31