
Reaction of 0.028 g of magnesium with excess hydrochloric acid generated 31.0 ml of hydrogen gas. the gas was collected by water displacement at 22 degree c. the barometric pressure in the lab was 746 mm hg. the vapor pressure of water at 22 degree c is 19.8 mm hg. use dalton's law to calculate the partial pressure of hydrogen gas in the gas-collecting tube. use the combined gas law to calculate the volume of hydrogen at stp. what is the theoretical number of moles of hydrogen that can be produced from 0.028g of mg?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, livigrace9004
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2


Chemistry, 22.06.2019 12:30, MrSavannahCat
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1
You know the right answer?
Reaction of 0.028 g of magnesium with excess hydrochloric acid generated 31.0 ml of hydrogen gas. th...
Questions in other subjects:










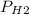 "
"
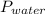
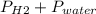
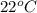 and 726.2 mm Hg and than convert it to STP conditions.
and 726.2 mm Hg and than convert it to STP conditions.
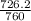
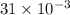 Litre
Litre
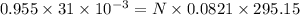
 moles
moles
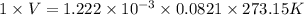
 (as 1 L = 1000 ml)
(as 1 L = 1000 ml)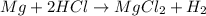
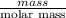
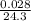
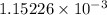 moles
moles


