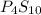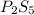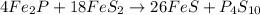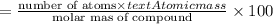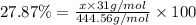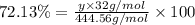Ferrophosphorus (fe2p) reacts with pyrite (fes2) producing iron(ii) sulfide and a compound that is 27.87% p and 72.13% s by mass and has a molar mass of 444.56 g/mol.
a. determine the empirical and molecular formulas of this compound.
b. empirical formula: molecular formula:
c. write a balanced chemical equation for this reaction. do not include phases.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:30, 91miketaylor
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 21:50, SoccerAllStar2
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
Ferrophosphorus (fe2p) reacts with pyrite (fes2) producing iron(ii) sulfide and a compound that is 2...
Questions in other subjects:

History, 08.12.2020 01:00


Biology, 08.12.2020 01:00

Chemistry, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00




Biology, 08.12.2020 01:00

History, 08.12.2020 01:00