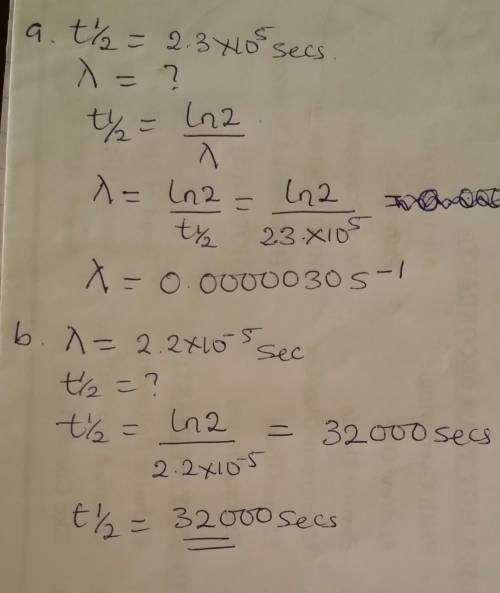Chemistry, 20.01.2020 23:31 ayoismeisjuam
The gas-phase decomposition of so2cl2, so2cl2(g)→so2(g)+cl2(g), is first order in so2cl2. at 600 k the half-life for this process is 2.3×105s.
part a
what is the rate constant at this temperature?
express your answer using two significant figures.
part b
at 320 ∘c the rate constant is 2.2×10−5s−1. what is the half-life at this temperature?
express your answer using two significant figures.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:50, britotellerialuis
Evaluate this exponential expression,8. (2 + 3)2 – 42
Answers: 3

Chemistry, 21.06.2019 21:10, danielahchf
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 10:30, mv603177
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1
You know the right answer?
The gas-phase decomposition of so2cl2, so2cl2(g)→so2(g)+cl2(g), is first order in so2cl2. at 600 k t...
Questions in other subjects: