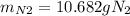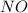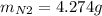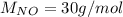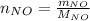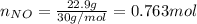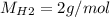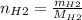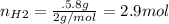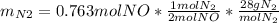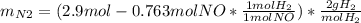Chemistry, 20.01.2020 23:31 jewlbug4358
For the following reaction, 22.9 grams of nitrogen monoxide are allowed to react with 5.80 grams of hydrogen gas. nitrogen monoxide (g) + hydrogen (g)> nitrogen (g) + water (1) what is the maximum amount of nitrogen gas that can be formed? what is the formula for the limiting reagent? grams what amount of the excess reagent remains after the reaction is complete? grams

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, aidengalvin20
One or more substances changing into one or more substances is an example of a
Answers: 1


You know the right answer?
For the following reaction, 22.9 grams of nitrogen monoxide are allowed to react with 5.80 grams of...
Questions in other subjects:








History, 02.09.2020 18:01

Biology, 02.09.2020 18:01