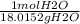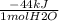Chemistry, 20.01.2020 21:31 fansofboys
Suppose that 0.88g of water condenses on a 75.0-g block of iron that is initially at 22 degrees celsius. if the heat released during condensation goes only to warming the block, what is the final temperature (in degrees celsius) of the iron block? assume a constant enthalpy of vaporization for water of 44.0 kj/mol.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, reeceslife481
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1


Chemistry, 22.06.2019 23:00, poolwaterisgross
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

You know the right answer?
Suppose that 0.88g of water condenses on a 75.0-g block of iron that is initially at 22 degrees cels...
Questions in other subjects:




World Languages, 27.06.2019 00:00


English, 27.06.2019 00:00