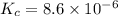Chemistry, 20.01.2020 21:31 amandanunnery33
Titanium(iv) chloride decomposes to form titanium and chlorine, like this: (l)(s)(g) at a certain temperature, a chemist finds that a reaction vessel containing a mixture of titanium(iv) chloride, titanium, and chlorine at equilibrium has the following composition: compound amount calculate the value of the equilibrium constant for this reaction. round your answer to significant digits.

Answers: 1


Other questions on the subject: Chemistry



You know the right answer?
Titanium(iv) chloride decomposes to form titanium and chlorine, like this: (l)(s)(g) at a certain t...
Questions in other subjects:





Engineering, 29.10.2020 06:00

English, 29.10.2020 06:00

Geography, 29.10.2020 06:00

Mathematics, 29.10.2020 06:00


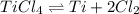
 4.18 g
4.18 g 1.08g
1.08g the value of the equilibrium constant for this reaction.
the value of the equilibrium constant for this reaction.
![[TiCl_4]=\frac{4.18 g}{190 g/mol\times 5.2 L}=0.004231 mol/L](/tpl/images/0463/1030/e0fe7.png)
![[Ti]=\frac{1.32 g}{48 g/mol\times 5.2 L}=0.005288 mol/L](/tpl/images/0463/1030/9e0bf.png)
![[Cl_2]=\frac{1.08 g}{71 g/mol\times 5.2 L}=0.002925 mol/L](/tpl/images/0463/1030/2b02a.png)
![K_c=[Cl_2]^2](/tpl/images/0463/1030/ae7ed.png)