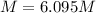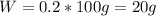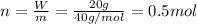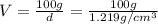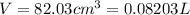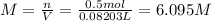Chemistry, 20.01.2020 20:31 madams4450
A20.0%(m/m) solution of naoh (fm 40.00) has a density of 1.219 g/cm'. calculate the solution's molarity. (6.10 m) 2. * 10 w= amountiof solute d- density of solution motanty m= molecular mass ot solute %3d

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, nadiarose6345
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 15:40, alleshia2007
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
A20.0%(m/m) solution of naoh (fm 40.00) has a density of 1.219 g/cm'. calculate the solution's molar...
Questions in other subjects:





Chemistry, 27.01.2020 23:31


History, 27.01.2020 23:31

Biology, 27.01.2020 23:31

Mathematics, 27.01.2020 23:31