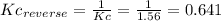Chemistry, 20.01.2020 19:31 naomijefferson22
Balance each of the following examples of heterogeneous equilibria and write each kc expression. then calculate the value of kc for the reverse reaction.
(1) al(s) + naoh(aq) + h2o(l) ⇋ na[al(oh)4](aq) + h2(g) kc for balanced reaction = 11
(2) h2o(l) + so3(g) ⇋ h2so4 (aq) kc for balanced reaction = 0.0123
(3) p4(s) + o2(g) ⇋ p4o6(s) kc for balanced reaction = 1.56

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, sleimanabir
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 21.06.2019 20:10, maribel2421
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 21.06.2019 21:00, tamikagoss22
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 05:00, Ashleyvasquez2261
Type the letter that represents the correct location for each particle type below.
Answers: 1
You know the right answer?
Balance each of the following examples of heterogeneous equilibria and write each kc expression. the...
Questions in other subjects:

Chemistry, 17.10.2020 20:01

English, 17.10.2020 20:01








 + 7 H_2(g)](/tpl/images/0462/9109/35a9e.png)
![Kc=\frac{[Na[Al(OH)_4]]^2*[H_2]^7}{[NaOH]^2}](/tpl/images/0462/9109/5fa1b.png)
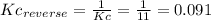
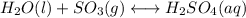
![Kc=\frac{[H_2SO_4]}{[SO_3]^2}](/tpl/images/0462/9109/da917.png)
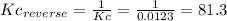
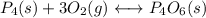
![Kc=\frac{1}{[O_2]^3}](/tpl/images/0462/9109/3ee9b.png)