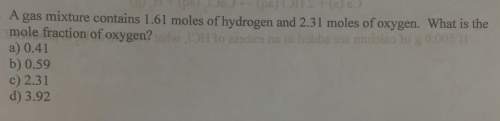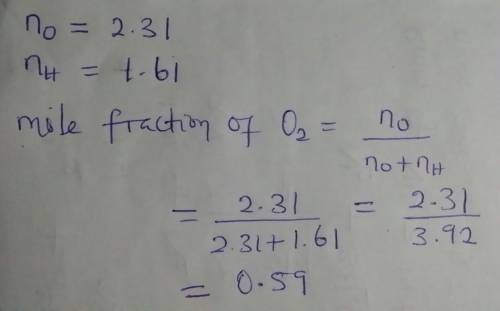Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, itsyaboiamo
Why should the scientific method be used to answer a question? a. it provides a way to test an idea without any bias. b. it provides a way to test a hypothesis. c. it provides a way to ensure all hypotheses are proven correct. d. it provides a way to quickly turn a hypothesis into a scientific theory.
Answers: 1


Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1
You know the right answer?
Agas mixture contains 1.61 moles of hydrogen and 2.31 moles of oxygen. what is the mole fraction of...
Questions in other subjects:


History, 11.11.2020 22:00

Mathematics, 11.11.2020 22:00

Mathematics, 11.11.2020 22:00




Biology, 11.11.2020 22:00

Mathematics, 11.11.2020 22:00