
Chemistry, 18.01.2020 15:31 nails4life324
Calculate the ph of a solution formed by mixing 20.00 ml of 0.30 m hcl with 30.00 ml of 0.15 m hno3? ?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, pinkycupcakes3oxbqhx
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 03:40, 19thomasar
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3


Chemistry, 23.06.2019 11:30, nadine6085859r
Which of the following is a property of nonmetals? a. nonmetals are ductile. b. nonmetals have a shiny luster. c. nonmetals have high density. d. nonmetals are nonconductors.
Answers: 1
You know the right answer?
Calculate the ph of a solution formed by mixing 20.00 ml of 0.30 m hcl with 30.00 ml of 0.15 m hno3?...
Questions in other subjects:

Mathematics, 30.05.2020 01:04

Chemistry, 30.05.2020 01:04






Mathematics, 30.05.2020 01:05

Mathematics, 30.05.2020 01:05

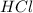 and
and 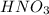 are strong acids which ionize completely in water. According to stoichiometry, 1 mole of each will produce 1 mole of hydronium cations. Therefore, let's calculate the total number of moles of hydronium and find the final concentration of it keeping in mind the dilution:
are strong acids which ionize completely in water. According to stoichiometry, 1 mole of each will produce 1 mole of hydronium cations. Therefore, let's calculate the total number of moles of hydronium and find the final concentration of it keeping in mind the dilution: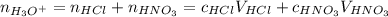
![[H_3O^+] = \frac{c_{HCl}V_{HCl} + c_{HNO_3}V_{HNO_3}}{V_{HCl} + V_{HNO_3}}](/tpl/images/0460/9459/33d70.png)
![pH = -\log[H_3O^+] = -\log(\frac{c_{HCl}V_{HCl} + c_{HNO_3}V_{HNO_3}}{V_{HCl} + V_{HNO_3}}) = -\log(\frac{0.30~M\cdot 20.00~mL + 0.15~M\cdot 30.00~mL}{20.00~mL + 30.00~mL}) = 0.68](/tpl/images/0460/9459/5204f.png)



