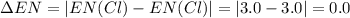Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:40, monkey2865
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 14:30, srutkowske1489
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
You know the right answer?
Consider the following electronegativity values:
h = 2.1, cl = 3.0, f = 4.0
which mole...
h = 2.1, cl = 3.0, f = 4.0
which mole...
Questions in other subjects:









Mathematics, 10.03.2020 17:59

History, 10.03.2020 18:00