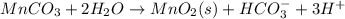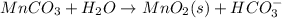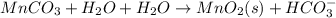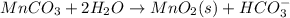Write a half-reaction for the oxidation of the manganese in mnco3(s) to mno2(s) in neutral groundwater where the carbonate-containing species in the product is hco3–(aq). add h2o and h+ to balance the h and o atoms in the equation. do not add electrons; you may leave the half-reaction unbalanced with respect to charge

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 20:20, Matseleng3775
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 22.06.2019 21:50, SoccerAllStar2
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
Write a half-reaction for the oxidation of the manganese in mnco3(s) to mno2(s) in neutral groundwat...
Questions in other subjects:




Biology, 16.01.2020 22:31



History, 16.01.2020 22:31



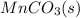 to
to 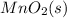 .:
.: