Chemistry, 18.01.2020 04:31 Mordred809
2. a neutralization reaction was carried out in a calorimeter. the change in temperature (at) of the solution was 5.6 °c and the mass of the solution was 100.0 g. calculate the amount of heat energy gained by the solution (qsol). use 4.18 j/(g. c) as the specific heat, cs, of the solution.
3. what is the value of qreaction for the neutralization reaction described in number 2?
4. how many moles of phosphoric acid are contained in 50.0 ml of 0.60 m h3po4?
5. what is the value of ahr kj/mol phosphoric acid) if 50.0 ml of 0.60 m reaction in h3po4 was used in the reaction described in number 2?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, dpazmembreno
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 05:30, alaynagrace1111
What is the mass of each element in a 324.8 sample of co2
Answers: 1
You know the right answer?
2. a neutralization reaction was carried out in a calorimeter. the change in temperature (at) of the...
Questions in other subjects:




Mathematics, 10.06.2021 02:20



Mathematics, 10.06.2021 02:20

Mathematics, 10.06.2021 02:20


Mathematics, 10.06.2021 02:20

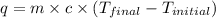
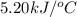
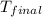 = final temperature
= final temperature 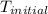 = initial temperature
= initial temperature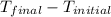 = 5.6°C
= 5.6°C 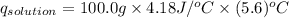
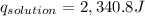
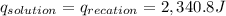
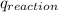 of reaction for the neutralization reaction described in number 2.
of reaction for the neutralization reaction described in number 2.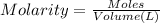
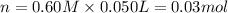
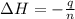
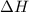 = enthalpy change = ?
= enthalpy change = ?