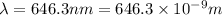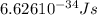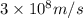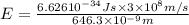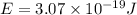When an electron in a 2p orbital of a particular atom makes a transition to the 2s orbital, a photon of approximate wavelength 646.3 nm is emitted. the energy difference between these 2p and 2s orbitals is: . 3.07 ã 10^â28 jb. 3.07 ã 10^â19 jc. 3.07 ã 10^â17 jd. 1.28 ã 10^â31 je. none of these

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, armahoney8566
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2


Chemistry, 22.06.2019 12:30, kaliyab191
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 15:30, elizabethprasad2
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
When an electron in a 2p orbital of a particular atom makes a transition to the 2s orbital, a photon...
Questions in other subjects:


Mathematics, 27.01.2020 06:31



Mathematics, 27.01.2020 06:31


Mathematics, 27.01.2020 06:31

Mathematics, 27.01.2020 06:31

Computers and Technology, 27.01.2020 06:31