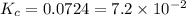Chemistry, 18.01.2020 00:31 honeylebling
Mercury(ii) oxide decomposes to form mercury and oxygen, like this: (s)(l)(g) at a certain temperature, a chemist finds that a reaction vessel containing a mixture of mercury(ii) oxide, mercury, and oxygen at equilibrium has the following composition:

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 05:50, mrylenastewart
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 23.06.2019 10:00, lexusdixon3
Which number should be placed before f2 on the reactants side equation to make equation balanced? xe + > xef4
Answers: 1
You know the right answer?
Mercury(ii) oxide decomposes to form mercury and oxygen, like this: (s)(l)(g) at a certain temperat...
Questions in other subjects:

English, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00

English, 11.11.2020 01:00


Mathematics, 11.11.2020 01:00


Mathematics, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00

English, 11.11.2020 01:00

History, 11.11.2020 01:00

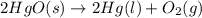
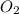 22.7 g
22.7 g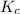 for this reaction. Round your answer to 2 significant digits.
for this reaction. Round your answer to 2 significant digits.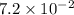
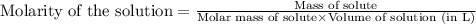
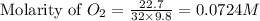
![K_c=[O_2]](/tpl/images/0460/0182/99fa8.png)