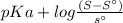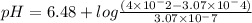Chemistry, 17.01.2020 23:31 bethzabezarahi
Sulfadiazine is a sulfa drug. the solubility of sulfadiazine is 3.07x10-4 mol dm-3 (as acid form). the pk a of the acid form is 6.48 given a solution of sulfadiazine-sodium (salt) of 4x10-2 mole dm-3 concentration, calculate at what ph starts the precipitation of the acid form

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, JKINGblackstar3502
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1


Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
Sulfadiazine is a sulfa drug. the solubility of sulfadiazine is 3.07x10-4 mol dm-3 (as acid form). t...
Questions in other subjects:

Spanish, 09.12.2020 03:30

English, 09.12.2020 03:30

Mathematics, 09.12.2020 03:30

Mathematics, 09.12.2020 03:30

Arts, 09.12.2020 03:30


Business, 09.12.2020 03:30

Mathematics, 09.12.2020 03:30

History, 09.12.2020 03:30

Social Studies, 09.12.2020 03:30