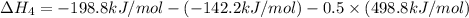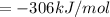Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, ashiteru123
Someone, part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 1

Chemistry, 22.06.2019 05:00, hjamya17
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2
You know the right answer?
Using hess's law, what is îhâ°rxn for the following reaction? no(g) + o(g) â no2(g)no(g) + o3(g) â n...
Questions in other subjects:

Business, 20.11.2020 19:50

Health, 20.11.2020 19:50

Health, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50






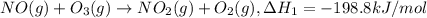 ..[1]
..[1]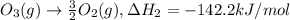 ..[2]
..[2]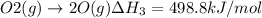 ..[3]
..[3]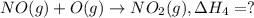 ..[4]
..[4]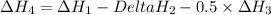 (By using Hess's law)
(By using Hess's law)