Consider the following reaction:
a + b + c right arrow d
the rate law for...

Consider the following reaction:
a + b + c right arrow d
the rate law for this reaction is as follows:
rate = k time fraction [a][c]^2 over [b]^1/2
suppose the rate of the reaction at certain initial concentrations of a, b, and c is 1.12×10-2 m/s.
what is the rate of the reaction if the concentrations of a and c are doubled and the concentration of b is tripled?
rate 2 = ? m/s

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 06:00, joelpimentel
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 08:30, waterborn7152
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 12:30, johnsont8377
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
You know the right answer?
Questions in other subjects:


Geography, 19.05.2020 22:09

Mathematics, 19.05.2020 22:09


Mathematics, 19.05.2020 22:09


History, 19.05.2020 22:09

Advanced Placement (AP), 19.05.2020 22:09


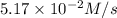
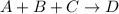
![\text{Rate}_1=k\frac{[A][C]^2}{[B]^{1/2}}](/tpl/images/0459/9288/92a79.png)
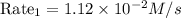
![\text{Rate}_2=k\frac{[2A][2C]^2}{[3B]^{1/2}}\\\\\text{Rate}_2=\frac{2\times 2^2}{3^{1/2}}\times (k\frac{[A][C]^2}{[B]^{1/2}})\\\\\text{Rate}_2=4.62\times (\text{Rate}_1)\\\\\text{Rate}_2=4.62\times 1.12\times 10^{-2}\\\\\text{Rate}_2=5.17\times 10^{-2}M/s](/tpl/images/0459/9288/30eea.png)



