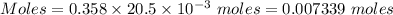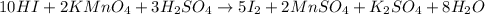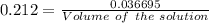Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, awdadaddda
How air particles exert a pressure on the inside of the balloon
Answers: 1


Chemistry, 22.06.2019 04:00, BaileyElizabethRay
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
You know the right answer?
How many milliliters of a 0.212 m hi solution are needed to reduce 20.5 ml of a 0.358 m kmno4 soluti...
Questions in other subjects:

English, 29.09.2019 23:50

Health, 29.09.2019 23:50

Health, 29.09.2019 23:50

Biology, 29.09.2019 23:50



Chemistry, 29.09.2019 23:50



Biology, 29.09.2019 23:50

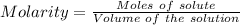
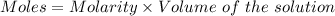
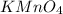 :
: