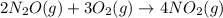Chemistry, 17.01.2020 02:31 kayabwaller4589
In the following reaction, what is the relationship between the rate at which the nitrous oxide is used up, the rate at which the oxygen is used, and the rate at which the nitrogen dioxide is produced? 2n2o(g)+ 3o2(g) → 4no2(g)

Answers: 1


Other questions on the subject: Chemistry



You know the right answer?
In the following reaction, what is the relationship between the rate at which the nitrous oxide is u...
Questions in other subjects:

Social Studies, 12.12.2021 06:20

Mathematics, 12.12.2021 06:20


English, 12.12.2021 06:20


Social Studies, 12.12.2021 06:20




Geography, 12.12.2021 06:20

![\text{Rate of disappearance of }N_2O=-\frac{1}{2}\frac{d[N_2O]}{dt}](/tpl/images/0458/6405/68f0b.png)
![\text{Rate of disappearance of }O_2=-\frac{1}{3}\frac{d[O_2]}{dt}](/tpl/images/0458/6405/655e6.png)
![\text{Rate of formation of }NO_2=+\frac{1}{4}\frac{d[NO_2]}{dt}](/tpl/images/0458/6405/2ef8c.png)
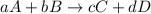
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0458/6405/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0458/6405/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0458/6405/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0458/6405/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0458/6405/d4b94.png)