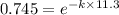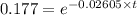Chemistry, 17.01.2020 02:31 fseftrwer6750
In a first-order decomposition reaction. 25.5% of a compound decomposes in 11.3 min. how long (in min) does it take for 82.3% of the compound to decompose?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, salvadorperez26
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 11:30, ansuaprajita1506
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 11:40, tatemelliott
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

You know the right answer?
In a first-order decomposition reaction. 25.5% of a compound decomposes in 11.3 min. how long (in mi...
Questions in other subjects:




Mathematics, 18.03.2021 20:20




Mathematics, 18.03.2021 20:20

Mathematics, 18.03.2021 20:20

Mathematics, 18.03.2021 20:20

![[A_t]=[A_0]e^{-kt}](/tpl/images/0458/6502/1ef89.png)
![[A_t]](/tpl/images/0458/6502/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0458/6502/9a686.png) is the initial concentration
is the initial concentration
![\frac {[A_t]}{[A_0]}](/tpl/images/0458/6502/0d33c.png) = 1 - 0.255 = 0.745
= 1 - 0.255 = 0.745![\frac {[A_t]}{[A_0]}=e^{-k\times t}](/tpl/images/0458/6502/16cf4.png)