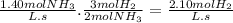Chemistry, 16.01.2020 23:31 kylabreanne120
If the average rate of appearance of nh3 in the following reaction is 1.40 m/s, what is the average rate of disappearance of h2 during the same time period?
n2(g) + 3h2(g) → 2nh3(g)
a. 5.40 m/s
b. 2.80 m/s
c. 1.20 m/s
d. 0.700 m/s
e. 2.10 m/s

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2


Chemistry, 22.06.2019 21:00, estherdinhllama
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1
You know the right answer?
If the average rate of appearance of nh3 in the following reaction is 1.40 m/s, what is the average...
Questions in other subjects: