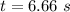Chemistry, 16.01.2020 23:31 jayjinks976
For the simple decomposition reactionab(g)→ a(g) + b(g)rate =k[ab]2 and k=0.2 l/mol*s . how long will it takefor [ab] to reach 1/3 of its initial concentration 1.50 mol/l?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 17:30, llamasking
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 19:30, youngdelvin123
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
For the simple decomposition reactionab(g)→ a(g) + b(g)rate =k[ab]2 and k=0.2 l/mol*s . how long wil...
Questions in other subjects:










![Rate = k[AB]^2](/tpl/images/0458/3241/7f1de.png)
![\frac{1}{[A_t]} = \frac{1}{[A]_0}+kt](/tpl/images/0458/3241/f2ee3.png)
![[A_0]](/tpl/images/0458/3241/9a686.png) is the initial concentration = 1.50 mol/L
is the initial concentration = 1.50 mol/L![[A_t]](/tpl/images/0458/3241/5262c.png) is the final concentration = 1/3 of initial concentration =
is the final concentration = 1/3 of initial concentration = 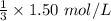 = 0.5 mol/L
= 0.5 mol/L