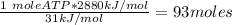Chemistry, 16.01.2020 05:31 JayLiz1737
Ask your teacher referring to the metabolic process below, calculate the maximum number of moles of atp that can be synthesized from adp from the breakdown of one mole of glucose. c6h12o6(s) + 6 o2(g) → 6 co2(g) + 6 h2o(l) δg° = −2880 kj/mol adp + h3po4 → atp + h2o δg° = 31 kj/mol webassign will check your answer for the correct number of significant figures. moles

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, fgcherubin
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

You know the right answer?
Ask your teacher referring to the metabolic process below, calculate the maximum number of moles of...
Questions in other subjects:

Mathematics, 24.08.2020 17:01



English, 24.08.2020 17:01


Mathematics, 24.08.2020 17:01




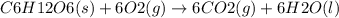 ΔG°=-2880 KJ/mol
ΔG°=-2880 KJ/mol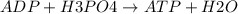 ΔG°=31 KJ/mol
ΔG°=31 KJ/mol