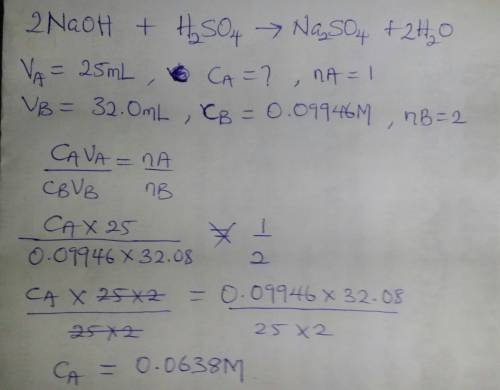Chemistry, 16.01.2020 04:31 risolatziyovudd
Asolution of sulfuric acid is titrated with a solution of sodium hydroxide to the phenolphthalein end point. the sample of sulfuric acid is 25.00 ml. the titration takes 32.08 ml of 0.09946 m sodium hydroxide.
a.) write out a balanced equation for the reaction.
b.) calculate the molarity of the sulfuric acid.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:10, amuijakobp78deg
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1

Chemistry, 22.06.2019 03:00, bchagnard2122
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 15:30, abdullaketbi71
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
Asolution of sulfuric acid is titrated with a solution of sodium hydroxide to the phenolphthalein en...
Questions in other subjects:



Health, 12.02.2020 03:17


Mathematics, 12.02.2020 03:17


Social Studies, 12.02.2020 03:17

Mathematics, 12.02.2020 03:17